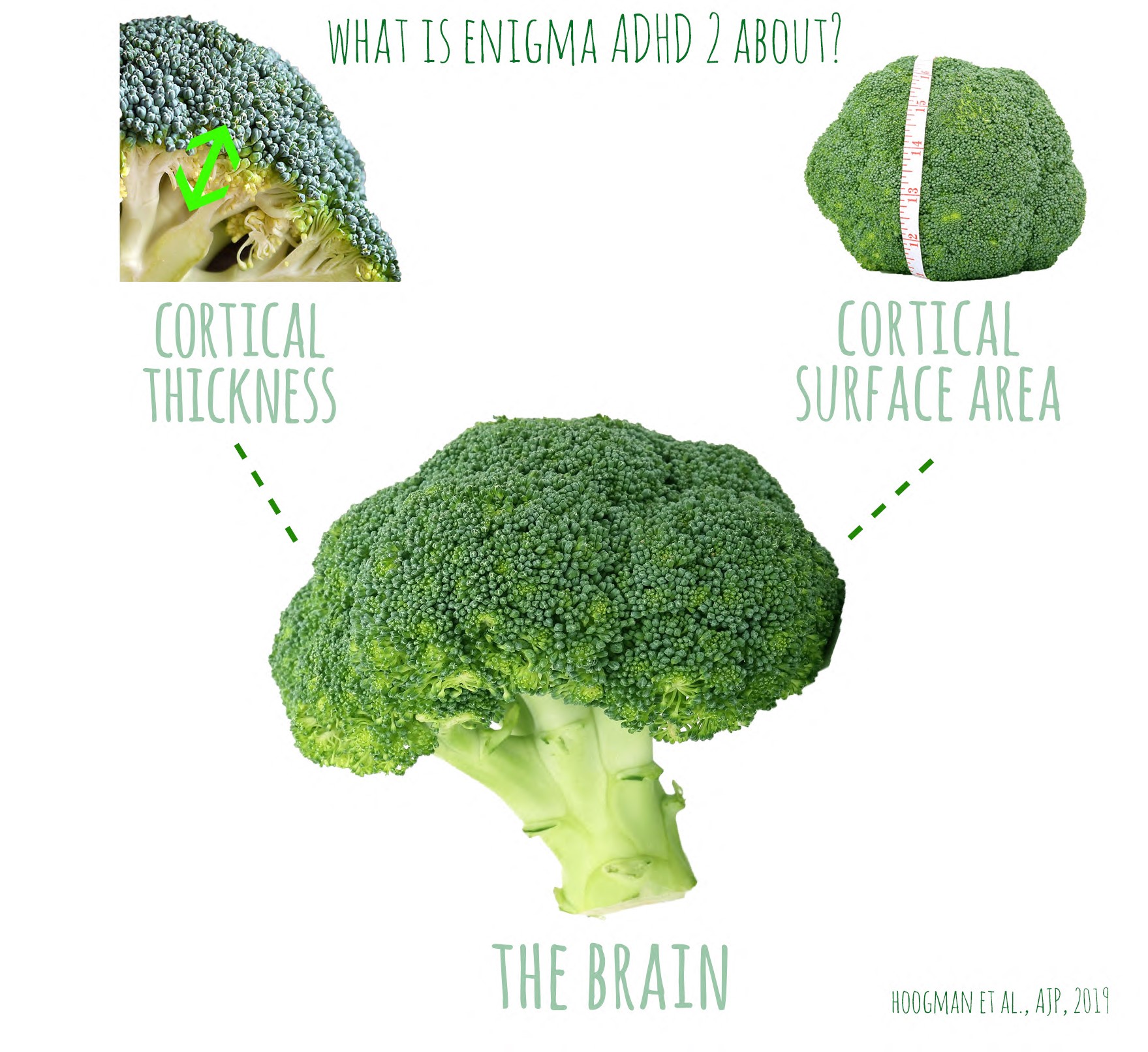
Genes play a big role in determining the architecture of our brain: the way it’s folded, the thickness of the outer layer, and the way different brain areas are connected. By combining data from all over the world, a large collaboration of researchers from the ENIGMA consortium has now identified almost 200 genetic variants that are involved in this brain architecture. These findings can help us to further understand the genetics of brain disorders.
Our genes contain the blueprint of our bodies. They contain information about how our cells function, and they determine for instance the colour of our eyes and hair, or whether we like cilantro (coriander) and bitter tastes. For some traits we know very well how they are influenced by genes. Eye color for instance is coded by only a few genes. But for many other traits such as height and personality, many different genes are involved. In addition, other (non-genetic) factors also influence these traits, such as malnutrition that can cause stunted growth.
The architecture of the brain is influenced by a large numer of genes, of which we still know very little. To investigate this, researchers combined genetic data of over 50.000 individuals with MRI-data. MRI-scans can show in detail the thickness of the outer layer of the brain, where all the brain cells are (also called the grey matter). They can also be used to measure how much this layer is folded, which gives information about the total surface of this outerlayer. This brain architecture is unique to every individual. The extent of the folds and the thickness of the outer layer have previously (in other research studies) been linked to cognitive abilities and various neurological and psychiatric disorders, such as Alzheimer’s disease, schizophrenia, depression, autism, and ADHD. It is therefore helpful to understand the genetics of this architecture, because it will help us to better understand the genetic mechanisms of these conditions.
The findings from this research study are also explained in this video:
This important research can only be done by combining a lot of data and collaborating with a large number of scientists and institutes. The ENIGMA consortium has been set upt to facilitate this kind of world-wide collaboration. The research that has now been published is the combined effort of more than 360 scientists from 296 departments across 184 different institutions and universities. They also made their results downloadable so that everyone who is interested can have a closer look.
The full publication can be found here: https://science.sciencemag.org/content/367/6484/eaay6690
See also our previous blogposts about these topics:
- Large genetic studies on ADHD: https://mind-the-gap.live/2019/07/17/no-i-do-not-have-adhd-i-am-just-busy-but-still-very-interesting-for-genetic-studies/
- The cortex and ADHD (another example of research from the ENIGMA consortium): https://mind-the-gap.live/2019/05/03/the-cortex-and-adhd-the-second-project-of-the-enigma-adhd-collaboration/
- Connectivity between brain regions and ADHD: https://mind-the-gap.live/2016/12/29/connectivity-adhd-brain/
- What it’s like to participate in an MRI experiment: https://mind-the-gap.live/2020/02/08/on-a-coalmine-and-an-mri-scanner-is-it-fun-participating-in-delta/